Day :
- Session -1
Human Vaccines - Infectious & Non Infectious Diseases | Vaccine Research & Development | Childhood Vaccines | Vaccination for pregnant women
Location: Hall - Linate

Chair
Frederic J Deschamps
University Hospital of Reims, France
Session Introduction
Randa S Hamadeh
Ministry of Public Health, Lebanon
Title: Polio: An international threat
Time : 12:00-12:25

Biography:
Randa S Hamadeh, a graduate of the American University of Beirut (AUB), is the head of the Primary Health Care and Social Health department, and the manager of Immunization and Essential Drugs Program at the Lebanese Ministry of Public Health. She is the coordinator of the Universal Health Coverage Project run jointly with the World Bank. She holds an MPH degree, a PhD in Public Health, and a Vaccinology Diploma. She contributed to creating a PHC network in Lebanon through which preventive programs and community health initiatives are initiated, usually involving local municipalities and NGOs. Dr. Hamadeh contributed to the introduction of the PHC facility accreditation program in Lebanon in 2008, and is the vice chair of the national accreditation committee. Dr. Hamadeh has actively participated in the foundation of various NGOs. She is the author of many public health papers and booklets. She is the preceptor of AUB Public Health students joining the PHC department for their residency programs since 2011. She contributes yearly as a lecturer and a panelist to International conferences in Public Health and Vaccines and she is an ADVAC(Advanced vaccinologist) member and an active participant in SAGE(Strategic Advisory Group of Experts) Global meetings. Dr. Hamadeh contributed to the partnership created among the ministry of Health and the Academia. She is a member of various National vaccines and Public Health technical committees. She participated in her capacity as a Primary Healthcare expert and a lecturer in the 36th anniversary of ALMA ATA Declaration in 2012. Dr. Hamadeh was elected for a Public Health Excellence Award, Delta Omega, Honorary Society of Public Health from the American University of Beirut in June 2017. She was nominated for the UNHCR Nansen Refugee Award in 2014 and was also nominated for Randa Bdeir Leadership Award at AUB for 2017. She was recently nominated to be the “Ambassador of Health in the Arab Countries” by the Arab League and the Arab Hospital Federation.
Abstract:
On 8 June 2017, World Health Organization announced an outbreak of polio in the Syrian Arab Republic. As at 16 January 2018, 74 cases have been confirmed as part of the outbreak. The median age of those infected is 15 months. This is the second outbreak of poliovirus to hit Syria since the onset of its humanitarian crisis in 2011. Unlike the first wild poliovirus outbreak in 2013, the current outbreak is a result of circulating vaccine-derived poliovirus type-2 (cVDPV2). Circulating vaccine-derived poliovirus can occur in rare instances when population immunity against polio is very low. In these settings, the weakened virus found in the oral polio vaccine (OPV) can spread between under-immunized individuals and over time, mutate into a virulent form that can cause paralysis. The only way to stop transmission of vaccine-delivered poliovirus is an immediate immunization response, the same as with any outbreak of wild polio. With high levels of population immunity, the virus will no longer be able to survive and the outbreak will come to close. Since confirmation of the cVDPV2 outbreak, there has been a concerted effort by outbreak response partners in conjunction with the Government of Syria and local authorities to stop transmission. WHO and UNICEF have jointly undertaken two mass vaccination rounds in affected areas. Both rounds were completed with more than 395,000 children vaccinated. In addition, targeted vaccination activities have also taken place in high risk areas of the country. The second phase of the outbreak response is currently underway. To facilitate the response, social mobilization teams have deployed to visit community leaders, pediatricians, local council workers and camp managers, and are conducting house-to-house visits to raise community awareness of the need for vaccination. Activities to strengthen surveillance for acute flaccid paralysis (AFP) – a major indicator for polio – are ongoing across the country. Contact sampling from all AFP cases continues and stool samples are being taken from healthy children arriving from known infected areas as well as form silent districts (districts that have not reported AFP cases in 2017). This situation urged neighboring countries to take immediate action. Lebanon is one of those countries most affected by the Syrian crisis due to geographical and cultural aspects that link the two countries. Lebanon, and since the onset of the Syrian crisis, has received more than 2M displaced and the displacement movement is ongoing even at a lower rate, the thing that necessitated the initiation of vaccination points at border line areas and at UNHCR registration centers in addition to ongoing national campaigns targeting 0-5 children regardless of nationality and of previous doses to maintain Lebanon polio free. Those activities became more focused nowadays targeting low coverage areas or areas of high density population where displaced has settled especially after April 2017 where bOPV was globally introduced to replace tOPV. To close, Polio is an international threat, one case of polio anywhere is polio everywhere. International communities should take immediate action toward securing more funds to guarantee vaccines availability and to channel more support to the health sector in general.
Catherine Heffernan
Public Health England, UK
Title: Perspectives of vaccinators on the factors affecting uptake of MenACWY vaccine amongst school leavers in London
Time : 12:25-12:50

Biography:
Catherine Heffernan is Principal Advisor for Commissioning Immunization and Vaccination Services at NHS England, London Region, England. She is a Consultant in Public Health and has spent the past five years as the London Regional Lead for Immunizations at Public Health England, advising NHS England, London Region on commissioning section 7a immunization programme. She is also an accredited educational supervisor for public health registrars and GP trainees and is an honorary senior clinical lecturer at London School of Hygiene and Tropical Medicine.
Abstract:
Statement of the Problem: Uptake of MenACWY amongst school leavers (18 years old) is suboptimal in London (9.9% compared to 17.4% nationally in 2015/16). There are studies looking at acceptability of MenACWY amongst adolescents (mostly younger adolescents as they receive the vaccination in schools) but very few studies look at how vaccination service provision may affect uptake of adolescent vaccination. This study identifies service delivery barriers and elicits insights from general practice staff on their interaction with this cohort. The purpose was to inform the NHSE (London) public health commissioning team’s strategy to improve MenACWY vaccination uptake in London.
Methodology & Theoretical Orientation: Qualitative semi-structured interviews study. Purposive sampling of practice nurses from three general practices from each of the three London clinical commissioning group areas (Barnet, Camden and Newham) with the largest numbers of 18-20 year old registered patients. Participants were recruited through their practice managers. A thematic analysis approach was used.
Findings: A total of ten interviews were conducted between June and August 2017. Five themes were identified: nurses unsupported by practice systems; difficulty getting school leavers into the practice; confused messaging; reliance on parental responsibility for health and; perception of complacency amongst adolescents.
Conclusion & Significance: Little is known about the service factors that impede uptake of adolescent vaccinations. This study shows that existing programmatic mechanisms for delivering the MenACWY catch-up programme were not adequate. The number of adolescent vaccinations offered has increased in the UK in the last five years and is likely to continue. General practice staff needs more systematic guidance on their role and how they can support vaccine decision-making in later adolescence.
Carmen Alvarez-Dominguez
Instituto de Investigación Marqués de Valdecilla, Spain
Title: Nanovaccines to prevent neonatal listeriosis
Time : 13:40-14:05

Biography:
Carmen Alvarez Dominguez has completed her PhD in Immunology, 1993 and has her expertise in listeriosis and Listeria based vaccines and nanovaccines for biomedical purposes. Her group has prepared different vaccines for listeriosis, either systemic listeriosis or neonatal listeriosis, using different vectors such as dendritic cells or nanoparticles. Moreover, they have also prepared Listeria-based nanovaccines as therapeutic tools for solid tumours. She has built this vaccine expertise after more than 27 years of experience in research, evaluation and teaching in hospital, basic research and academic institutions in Spain and USA. She is also moving recently to consultancy companies to put new vaccines into the market.
Abstract:
Clinical cases of neonatal listeriosis are associated with brain disease and fetal loss due to complications in early or late pregnancy, which suggests that microglial function is altered. This is believed to be the first study to link microglial apoptosis with neonatal listeriosis and listeriosis-associated brain disease, and to propose a new nanovaccine formulation that reverses all effects of listeriosis and confers Listeria monocytogenes (LM) specific immunity. We examined clinical cases of neonatal listeriosis in 2013–2015 and defined two useful prognostic immune biomarkers to design listeriosis vaccines: high anti-GAPDH1-22 titres and tumor necrosis factor (TNF)/interleukin (IL)-6 ratios. Therefore, we developed a nanovaccine with gold glyco-nanoparticles conjugated to short LM peptides and formulated with a pro-inflammatory Toll-like receptor 2/4-targeted adjuvant. The neonates born to nanovaccinated pregnant mice’s with listeriosis, showed brain and vascular disease and significant microglial dysfunction by induction of TNF-α-mediated apoptosis. This programmed TNF-mediated suicide explains LM dissemination in brains and livers and blocks production of early pro-inflammatory cytokines such as IL-1β and interferon-α/β. In contrast, neonates born to nanovaccinated mothers before LM infection, did not develop listeriosis or brain diseases and had functional microglia. In nanovaccinated mothers, immune responses shifted towards Th1/IL-12 pro-inflammatory cytokine profiles and high production of anti-LM antibodies, suggesting good induction of LM-specific memory.
Pragya Sharma
Maulana Azad Medical College, India
Title: Implications of IPV introduction in national immunization schedule and strategies to combat shortages
Time : 14:05-14:30

Biography:
Pragya Sharma has completed her MD in Public Health from Post Graduate Institute of Medical Sciences, Rohtak, India. After completing her education, she has served in Central Health Services, Government of India as Public Health Specialist heading the Reproductive and Child Health Programme for the National Capital Territory of Delhi, India for a period of three years. She is Professor in Community Medicine in Maulana Azad Medical College, a premier medical academic organization in the country. She has published more than 15 papers in reputed journals and has been serving as a reviewer member of repute.
Abstract:
With the upcoming globally synchronized switch from t OPV to b OPV for commitment of countries to be free from t OPV, 156 countries have pledged to stop using the same and replace it either with b OPV and IPV combination or only IPV. Thus there is humongous demand of IPV against the production capacity of the vaccine across the globe. There is an urgent need to explore various strategies to combat the forthcoming crisis in the given demand supply shortage and also in the post eradication era. Also it is important to consider the cost effective strategies for the middle and low income countries for sustainability once IPV has been included in the programme.
Helen Bright
Medimmune, UK
Title: Investigations into the reduced effectiveness of the H1N1pdm09 component of the live attenuated influenza vaccine
Time : 14:30-14:55

Biography:
Helen Bright is an Immunologist with over 20 years’ experience in the biopharmaceutical industry. She has a degree in Microbiology from the University of Newcastle upon Tyne and a PhD in RSV Vaccine Research. She is currently a Principal Scientist at Medimmune, responsible for the research, selection and development of vaccine strains for the seasonal and pandemic live attenuated influenza vaccine. Previously, she successfully led anti-viral, immune modulation and vaccine discovery projects at GSK and Pfizer. She has been a Reviewer for the MRC Infection and Immunity Board and a Journal Referee.
Abstract:
Decreased effectiveness of the influenza A(H1N1)pdm09 strains (A/California/7/2009 and A/Bolivia/559/2013) included in live attenuated influenza vaccines (LAIV) have been observed in recent years. Multiple hypotheses have been suggested as potential explanations for this reduced effectiveness compared with inactivated influenza vaccines (IIV). The most frequently cited hypotheses include poor replicative fitness of the A(H1N1)pdm09 LAIV strains, vaccine–virus interference in the quadrivalent formulation, reduced LAIV replication due to preexisting anti-influenza immunity from prior influenza vaccinations, and poor thermostability of A(H1N1)pdm09 LAIV strains. We have systematically evaluated each of these hypotheses and initiated a multifaceted scientific investigation in to the causes of the recently observed reduced effectiveness of LAIV. Laboratory studies show that A/ California and A/Bolivia strains have reduced replication in a human alveolar cell line, primary human nasal epithelium air-liquid cultures. Data suggests that the underlying mechanism for this is likely to be multi-factorial. For example, the pdm09H1N1 LAIV strains have reduced binding to α2,6-linked sialic acid receptors (the primary receptor for influenza viruses in the human upper respiratory tract) and increased neuraminidase activity. Finally, ferret studies confirm that LAIV which replicate well in tissue culture are more effective in protecting from wild type influenza virus challenge.
- Session - 2
Antibiotics | Antibiotic Resistance: Opportunities and Challenges | Drug Discovery and Novel Delivery Technologies | Antibiotics for Emerging and Re-emerging Diseases | Antibiotics and Mechanism of Action
Location: Hall - Linate

Chair
Marek Chmielewski
Polish Academy of Sciences, Poland
Session Introduction
Klara Stensvag
University of Tromsø - The Arctic University of Norway, Norway
Title: Antibacterial and anti-biofilm activity of novel compounds of arctic marine origin
Time : 14:55-15:20

Biography:
Klara Stensvåg completed her PhD and Postdoctoral studies at Arctic University of Norway (UiT). She is a Professor in Marine Biotechnology at The Norwegian College of Fishery Science at UiT. She has published more than 44 papers in reputed journals and has been serving as an Editorial Board Member for reputed journals. She is the Head of the research group in Marine Bioprospecting. Her research concerns antimicrobial compounds and genes of marine origin as source of developing novel antibacterial compounds with new mechanisms of actions against antimicrobial resistant bacteria.
Abstract:
Nature is still a probable source of novel antibiotics since almost 70% of the drugs approved today are based on knowledge from natural sources. Since few new commercial antibiotics are approved during the last decades, the rather little studied marine environment and molecules thereof, are regarded as very interesting because of the close connection, often in symbiotic relationship, between different organisms and microorganisms to each other. Marine resources like invertebrates, microalgae, plants, marine bacteria and fungi together with biological rest raw material, are thus promising for exploring novel antimicrobials with unique antibacterial strategies and anti-biofilm properties. In this context, both marine proteins/peptides and secondary metabolites are interesting because of their bioactivities against antibiotic resistant human pathogenic bacteria in the first place which also can define novel targets in pathogenic bacteria. Secondly, their structural motives/pharmacophores might be usable to design novel synthetic marine natural products mimics with promising antimicrobial and antibiofilm properties. We have characterized several new classes of marine AMPs and explored their mode of action by different tests. The organisms that produce these bioactives are collected from the Arctic or/and sub-Arctic region and can be very diverse covering biological resources from microalgae to invertebrates. This talk will cover different approaches in bioprospecting that include characterization of mechanisms of actions, SAR studies and give examples of designed new marine mimicking molecules as candidates of novel lead compounds of antimicrobials and anti-biofilm active compounds. The talk will also refer to the recently established Centre for new Antibacterial Strategies (CANS) at UiT.
Haihong Hao
Huazhong Agricultural University, China
Title: Functional genomics and transcriptomics of virulent and multidrug resistant Escherichia coli of poultry origin
Time : 14:55-15:20
Biography:
Haihong Hao has completed his PhD from Huazhong Agricultural University and he is a Visiting Scientist from Iowa State University (2008-2009) and NCTR USFDA (2015- 2017). He is an Associate Professor of College of Veterinary Medicine at Huazhong Agricultural University. He has published more than 50 papers in reputed journals.
Abstract:
The pathogenic and multidrug resistant (MDR) Escherichia coli in poultry products may pose high risk to food safety. The MDR E. coli were selected by antimicrobial susceptibility tests. The Extended-Spectrum β-Lactamases (ESBL) were determined by antibiotics impregnated disks and double disk synergy test. The virulent characteristics, including the biofilm formation, the adhesion, invasion and survivability in Caco-2 and Raw 264.7, the median lethal dose (LD50) in two-day old chickens, were determined via in vitro and in vivo tests. The genes involved in MDR, ESBL and virulence were amplified by PCR. Six MDR E. coli isolates with higher virulence (27, 112, 130, 351, 357 and 381) were selected for next generation sequencing. The multidrug resistance genes (mdtE, F, G, and K) and genes encoding resistance to β-lactams, aminoglycosides, chloramphenicol, fosfomycin, fluoroquinolones and tetracycline were found in one MDR E.coli. The E.coli 381 with higher virulence in cell culture and 1000-fold more virulence in a chicken model than other strains. The Illumina HiSeq2500 transcriptome analysis found that multiple pathways involved in the resistance (e.g mdt for multidrug resistance, mate for efflux family, ompE for outer member, fsr for fosmidomycin resistance and ABC Transporters) and virulence (e.g inv for invasion, BssR and bdm for biofilm formation, NlpD for lipoprotein and ycr for two component systems) were up-regulated in E. coli 381. The results provided critical information about the highly virulent MDR E. coli strain of poultry origin, which can further be used for the development of prevention strategies and treatment procedures.
Iskakova Zhanar Baktybaevna
Kazakh University of Technology and Business, Kazakhstan
Title: Cytotoxic and antiradical activity of roseofungin
Time : 16:05-16:30

Biography:
Iskakova Zhanar Baktybaevna is a candidate of chemical sciences, Associate Professor. She is the Head of the Department of Science and Post-Graduate Education and; Scientific Secretary of the Kazakh University of Technology and Business. She has about 100 scientific publications, the author of one patent, three teaching aids, one electronic textbook and a monograph. She is a Laureate in the nomination Springer Nature top of the most published scientists of Kazakhstan in the field of Biological Sciences and Biomedicine. She is engaged in determining the biological activity of essential oils extracted from plants, extracts and substances in the Institute of Applied Chemistry.
Abstract:
The specialists in the Institute of Microbiology and Virology, Al-Farabi Kazakh National University and Nazarbayev University were engaged in the research and study of new antibiotics to increase the activity of producers of known antibiotics. Thus, a broad-spectrum antifungal polyene antibiotic, roseofungin was prepared for the treatment of deep and superficial mycoses. Physicochemical properties of roseofungin are investigated by L Vetlugina and R Dziubanova. Roseofungin is a mixture of two compounds differing only in one CH2-group. Due to the difficulty of separation and very similar properties, these compounds were not divided in a pure form; therefore a specific formula for roseofungin has not yet been established. The culture liquid of roseofungin used for research was represented by specialists of the National Centre of Biotechnology (Stepnogorsk, Kazakhstan). The determination of antiradical, cytotoxic activity is necessary to establish the presence of potential biological activity in pentaenoic antibiotics, which will allow them to be used as medicines in the future. The cytotoxic activity of the roseofungin was determined by the compressibility of Artemia salina. On the basis of the experiments, it was found that the roseofungin at concentrations of 10 and 5 mg/ml exhibits moderate toxicity - the mortality of larvae is 72%, and at a concentration of 1 mg/ml exhibits low toxicity, mortality - 48%. The antiradical activity of the roseofungin is determined by the reactions of the inhibition of 2, 2-diphyl-1-picrylhydrazyl radical (DPPH assay). The results showed that the roseofungin has a low antiradical activity in comparison with the standard butylhydroxyanisole.
Yuguang Mu
Nanyang Technological University, Singapore
Title: Binding modes of teixobactin to Lipid II: Molecular dynamics study
Time : 16:30-16:55

Biography:
Yuguang Mu has more than 20 years of experience in the area of Computational Biophysics. His main researches focuses on research fields, MD simulation method and data analysis method development, DNA dynamics, DNA protein, DNA-counterions interaction study, peptide, protein folding, unfolding study, especially aimed at folding, misfolding mechanism which could lead to amyloid fibril, RNA dynamics and folding study.
Abstract:
Teixobactin (TXB) is a newly discovered antibiotic, targeting the bacterial cell wall precursor Lipid II (LII). In the present work, four binding modes of TXB on LII were identified by a contact-map based clustering method. The highly flexible binary complex ensemble was generated by parallel tempering metadynamics simulation in a well-tempered manner (PTMetaD-WTE). In agreement with experimental findings, the pyrophosphate group and the attached first sugar subunit of LII are found to be the minimal motif for stable TXB binding. Three of the four binding modes involve the ring structure of TXB and have relatively higher binding affinities, indicating the importance of the ring motif of TXB in LII recognition. TXB-LII complexes with a ratio of 2:1 are also predicted with configurations such that the ring motif of two TXB molecules bound to the pyrophosphate-MurNAc moiety and the glutamic acid residue of one LII, respectively. Our findings disclose that the ring motif of TXB is critical to LII binding and novel antibiotics can be designed based on its mimetics.
Amin Noori
Social Security Hospital, Iran
Title: Imipenem and meropenem Drug Utilization Evaluation (DUE) in the social security hospital in Khorramabad
Time : 16:55-17:20

Biography:
Amin Noori completed his Graduation in Doctor of Pharmacy (Pharm D) in 2014. During his study in University he has worked in different research fields including Laboratory research, e-learning. He is still doing research on Clinical Trials, Bioinformatics, Chemonformatic and DUE. He is working in Social Security Hospital as Hospital Pharmacist in (Iranian Social Security Organization) since May 2016.
Abstract:
Background: Drug utilization assessment is the marketing, distribution, prescription and use of drugs in society with special importance on the resulting medical, social and economic costs (WHO). The purpose of DUR is to ensure drugs are used appropriately, safely and effectively to improve patient health. Carbapenems possess the broadest spectrum of activity and the greatest potency against Gram-positive and Gram-negative bacteria. Several recent studies clearly show that resistance to carbapenems is increasing throughout the world.
Methods: A descriptive cross-sectional study was performed during three periods: the summer and fall of 2016, spring and summer 2017, and fall and winter 2017. Imipenem and meropenem DUE were done in a hospital in Iran. All of the patients receiving imipenem and meropenem were enrolled in this study. AHFS drug protocols have been used to perform this study.
Results: The results of this study showed that the monitoring and evaluation of the drugs, imipenem and meropenem, reduced the expenditure, consumption and duration of drug intake during the three periods. The correct use of the drug was improved. During the 3rd period, the correct indication rose to 79% for imipenem and 76% for meropenem, but the correct dosages were only imipenem 24% and meropenem 32%. During the periods studied, empirical prescription decreased to imipenem, 58% and meropenem 64%.
Conclusion: Imipenem and meropenem are mostly administered empirically, rather than using an antibiogram. Although a lower empirical therapy would be desirable, the study improved the correct indication to 79%, and a reduction of the consumption period to 7.3.
- HIV Vaccines | Vaccine Research & Development | Human Vaccines - Infectious & Non Infectious Diseases | Veterinary Vaccines | Vaccines against Viral & Bacterial Diseases
Location: Hall - Linate

Chair
Chit Laa Poh
Sunway University, Malaysia
Session Introduction
Godwin W Nchinda
CIRCB, Cameroon
Title: Targeting conserved broadly neutralizing epitopes within HIV-1 envelope gp41 MPER as vaccine immunogens for seronegative partners of HIV-1 discordant couples
Time : 12:00-12:25

Biography:
Godwin Nchinda has his expertise in optimizing vaccine candidates against infectious diseases for preclinical and clinical evaluation in sub Saharan Africa. He has developed DNA, viral vectored and protein vaccines targeting the encoded HIV-1 vaccines to dendritic cells in situ. Dendritic cells are vital in initiating and regulating immune responses. He and his colleagues are pioneering a new generation of vaccines which are harnessing dendritic cells in situ to improve vaccine efficacy against infectious diseases. This strategy has been described in several peer reviewed publications with him as co-author.
Abstract:
Background: The membrane proximal external region (MPER) of HIV-1 envelope glycoprotein-41 (gp41) is targeted by several broadly neutralizing antibodies whose conserved linear epitopes are promising targets for vaccine design. However, a formidable challenge has remained the difficulty to design and deliver MPER based immunogens for the efficient induction of such broadly neutralizing HIV-1 specific antibodies (bnAb). This is mainly because the linear bnAb MPER epitopes are poorly accessible to the immune system. The overall objective of this study therefore was the development and validation of an RNA coliphage Qβ display system for efficient presentation of conserved bnAb epitopes to the immune system
Method: To overcome the challenge of effective presentation of MPER to the immune system we have selectively engineered the surface of the RNA coliphage Qβ to display a 51 aa consensus MPER peptide upon the surface of the phage particle. The expression cassettes were used for the production of QβMPER recombinant hybrid phages after transformation of HB101 strain.
Results: Specific recognition of some reported bnAb epitopes within MPER were confirmed in ELISA using the three recombinant QβMPER phages together with an MPER restrictive peptide as antigens and the bnAb 4E10, Z13e1, 2F5 and 10E8 as antibodies. Next the prevalence of MPER-specific antibodies was determined in plasma from long standing antiretroviral naïve HIV-1 infected participants of the CIRCB AFRODEC cohort. The greater majority (84%) of participants’ plasma showed MPER peptide specific reactivity with anti-MPER specific IgG antibody titers ranging from 200 to 409600 comparative to background IgG antibody titer. In immunogenicity studies in Balb/c mice the recombinant phages induced significantly high Anti-MPER-specific IgG antibody responses (P<0.04) in at least 60% of mice following three inoculations of each recombinant phage.
Conclusion: Thus, these novel recombinant QβMPER phages can be used to monitor MPER-specific immune responses in HIV-1 exposed or infected people. In addition the recombinant QβMPER phages could be used as immunogens either alone as demonstrated here in mice or in combination with other strategies for the induction of MPER specific immunity against HIV-1.
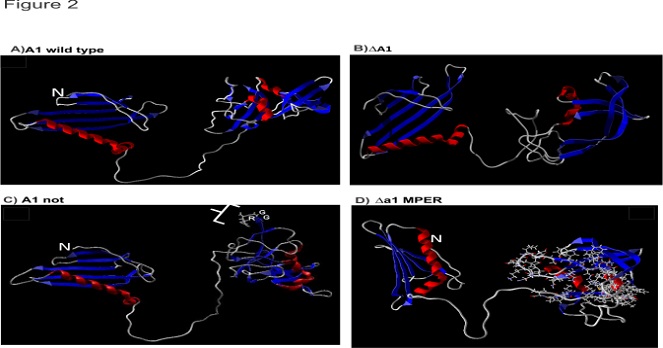
A) A1 wild type (B) ΔA1 (C) A1Not (D) ΔA1MPER (MPER highlighted in ball and stick rendering at the C terminus region of the protein). (N indicates the N terminus of the minor coat protein A1; red and blue colored structures indicate α-helix and β-sheets, respectively)
Xiao-Song He
VA Palo Alto Health Care System, USA
Title: Repeated influenza vaccination is a potential cause for reduced B cell response in the elderly
Time : 12:25-12:50

Biography:
Xiao-Song He has received his Doctorate degree from Fudan University, China and completed his Postdoctoral studies at Stanford University School of Medicine, USA. He is a Senior Research Scientist at VA Palo Alto Health Care System and Adjunct Professor at University of California in Davis, USA. He has published more than 60 original papers in the fields of Viral Immunology and Autoimmunity, and is a current member of the Editorial Advisory Board of The Journal of Infectious Diseases.
Abstract:
The disease burden of annual influenza epidemics is especially significant among elderly individuals. Although vaccination provides protection, influenza vaccine needs to be reformulated yearly due to the frequent mutations of circulating strains. Currently annual influenza vaccination is recommended for individuals aged six months or older. The protective efficacy of the inactivated influenza vaccine (IIV) is significantly lower in the elderly than the younger adults but the cause of this age effect is not fully understood. To address this issue, we investigated the circulating plasmablast response to IIV and found that the number of vaccine-induced plasmablasts, or activated B cells, was lower in the elderly than young adults. In addition, the numbers of de novo somatic hypermutations in the immunoglobulin genes of influenza-specific plasmablasts were also lower in the elderly, resulting in an antibody response poorly adapted to the new vaccine antigens. To further explore the cause of the reduced B cell response, we followed a cohort of individuals who received annual influenza vaccination in four consecutive years and measured their plasmablast response after each vaccination. The plasmablast response declined with increased number of vaccination, whereas the avidity of plasmablast-derived polyclonal antibodies did not increase with repeated immunization of the same influenza strain. Our findings suggest that repeated IIV immunization is a factor contributing to the reduced antibody response to influenza vaccination in the elderly who have extensive exposure to influenza antigens during their livetime. Therefore it is importance to develop universal influenza vaccines that do not require annual vaccination.
Mark Fife
The Pirbright Institute, UK
Title: Chicken IFITM gene knockout technology for enhanced vaccine production
Time : 13:40-14:05

Biography:
QTL and association studies), candidate gene mapping and molecular biology techniques. He has produced over 45 peer-reviewed publications and book chapters in this area before becoming a Group Leader at the Institute. His work has been the focus of extensive genome-wide and haplotype analysis using web-based SNP selector software that he has implemented at Pirbright. This work has culminated in the identification and characterization of several causal genes for important immune traits in chickens. His current research found a group of related genes called the interferon-inducible transmembrane (IFITM) genes that are able to prevent viruses from attacking and killing host cells. The aim of his group work is to determine the biology and genetic variation of these and similar immune genes in chickens; specifically, the ability of these genes to protect the host against avian viruses. The output of this work will be in identifying specific gene variants that correlate with resistance to a number of avian viruses, thus allowing poultry breeding programmes to select robust chickens, able to fight viral infections.
Abstract:
Type I interferon protect cells from viral infections through the induction of a group of genes collectively named interferon-stimulated genes (ISGs). Among these ISGs, are the IFITM (interferon-inducible transmembrane) which have been shown to restrict the replication of several highly pathogenic human viruses, including severe acute respiratory syndrome (SARS) coronavirus, filoviruses (Marburg virus and Ebola virus), influenza A viruses (IAVs), and flaviviruses (dengue virus). The genetics and genomics group have identified these antiviral proteins in the chicken (chIFITM) and have shown that a reduction in chIFITM expression results in an increase in the virus titre in CEFs infected with avian influenza A virus (AIV) H9N2, suggesting that chIFITMs have a functional role in the control of viral infections. The observation may have useful implications in terms of vaccine production. Many vaccines are produced in embryonated hen’s eggs or continuous avian cell lines. However, it is well established that the rate determining step in the manufacture of numerous vaccines is the induction of antiviral immune responses that prevents the replication of vaccine viruses. To generate chIFITM knock-down, we will use cutting edge genetic approaches such the CRISPR/Cas9 system which will directly target and knock-out chIFITM expression. We believe that this approach will overcome the rate limiting step in vaccine production, directly resulting in increased vaccine yields and improve the speed at which vaccines can be manufactured. We are currently in talks with major vaccine producers keen to adopt this internationally patented technology, to advance the field of both animal and human vaccine production. This work is being conducted in partnership with Horizon Discovery, using their extensive expertise in genetic modification using gene editing technologies. The broad objective of the project is to observe the effect the knock-out of chIFITM genes expression, achieved via CRIPSR/Cas9 transfection methods, has on viral titre in avian cell lines (commonly used for vaccine production) infected with influenza A virus. In addition, through analyzing the genetic material of a wide variety of chicken breeds and outlying avian species that differ in levels of resistance to these viruses, we hope to identify versions of these proteins that give protection, in laboratory, commercial and “backyard” chickens. Analysis of these proteins in the chicken presents opportunities not just for a greater understanding of viral resistance, but also as tools to combat viruses in the poultry farming. It may be feasible to selectively breed for birds with improved resilience to viral infections; however, this requires the identification of resistance-associated factors and knowledge of how they act.
Chit Laa Poh
Sunway University, Malaysia
Title: MicroRNA reduction of enterovirus 71 viral replication attenuates and confers protective immune response in mice
Time : 14:05-14:30

Biography:
Dr. Chit Laa Poh is a Distinguished Professor and Head of the Centre for Biomedical Sciences at Sunway University. She completed her PhD at Monash University (Australia) in 1980 and conducted short periods of postdoctoral training from the Pasteur Institute, Cambridge University and the University College London. She has previously worked in the Yong Loo Lin School of Medicine, National University of Singapore (NUS) for 25 years. She has published more than 85 papers in reputed journals and has been serving as an editorial board member of Journal of Biosceince and Bioengineering, Austin Journal of Tropical Medicine and Hygeine, Journal of Virology and Emerging Diseases and Annals of Translational Medicine and Epidemiology.
Abstract:
The hand, foot, and mouth disease (HFMD) is generally manifested as a subclinical infection, but fatal neurological complications can occur in young children. Epidemiological surveillance in China from 2008-2014 showed that 43.73% of HFMD cases were due to EV-A71. Up to date, there is no WHO-approved vaccine against EV-A71. This study demonstrated a novel miRNA vaccine construct for EV-A71 which decreased viral replication in vitro, whilst conferring a protective immune response in four-week old ICR mice. A vaccine construct was engineered to carry microRNA (miRNA) target sequences let-7a and miR-124a in the EV-A71 genome, allowing endogenous RNA silencing in specific cell types. The viral RNA copy number of the miRNA vaccine strain was much lower in RD (1.2 X 102) and SHSY-5Y cells (7.7 X 10) that expressed let-7a and miR-124a, respectively. The miRNA vaccine construct caused much reduction in plaque number (3.5 x 104 PFU/ml) in the SHSY-5Y cells as compared to the wild type virus (5.0 x 108 PFU/ml). No weight loss and hind limb paralysis were observed in the miRNA vaccine-administered mice (n=5) in comparison to the naïve group of mice (n=5). Significantly elevated systemic levels of IFN-γ and lower levels of pro-inflammatory cytokines TNF-α and IL-6 were detected. Higher CD8+ T cell response was elicited by the miRNA vaccine strain in mice as compared to the inactivated vaccine. The miRNA vaccine construct was able to confer protective immunity against EV-A71 sub-genotypes B3, C3 and C4. Ten serial passage studies to determine the genetic stability of the target sequences let-7a and miR-124a showed that the sequences did not revert to wild type virulence. Overall, the miRNA vaccine construct is an effective attenuation strategy for vaccine development.
