
Godwin W Nchinda
CIRCB, Cameroon
Title: Targeting conserved broadly neutralizing epitopes within HIV-1 envelope gp41 MPER as vaccine immunogens for seronegative partners of HIV-1 discordant couples
Biography
Biography: Godwin W Nchinda
Abstract
Background: The membrane proximal external region (MPER) of HIV-1 envelope glycoprotein-41 (gp41) is targeted by several broadly neutralizing antibodies whose conserved linear epitopes are promising targets for vaccine design. However, a formidable challenge has remained the difficulty to design and deliver MPER based immunogens for the efficient induction of such broadly neutralizing HIV-1 specific antibodies (bnAb). This is mainly because the linear bnAb MPER epitopes are poorly accessible to the immune system. The overall objective of this study therefore was the development and validation of an RNA coliphage Qβ display system for efficient presentation of conserved bnAb epitopes to the immune system
Method: To overcome the challenge of effective presentation of MPER to the immune system we have selectively engineered the surface of the RNA coliphage Qβ to display a 51 aa consensus MPER peptide upon the surface of the phage particle. The expression cassettes were used for the production of QβMPER recombinant hybrid phages after transformation of HB101 strain.
Results: Specific recognition of some reported bnAb epitopes within MPER were confirmed in ELISA using the three recombinant QβMPER phages together with an MPER restrictive peptide as antigens and the bnAb 4E10, Z13e1, 2F5 and 10E8 as antibodies. Next the prevalence of MPER-specific antibodies was determined in plasma from long standing antiretroviral naïve HIV-1 infected participants of the CIRCB AFRODEC cohort. The greater majority (84%) of participants’ plasma showed MPER peptide specific reactivity with anti-MPER specific IgG antibody titers ranging from 200 to 409600 comparative to background IgG antibody titer. In immunogenicity studies in Balb/c mice the recombinant phages induced significantly high Anti-MPER-specific IgG antibody responses (P<0.04) in at least 60% of mice following three inoculations of each recombinant phage.
Conclusion: Thus, these novel recombinant QβMPER phages can be used to monitor MPER-specific immune responses in HIV-1 exposed or infected people. In addition the recombinant QβMPER phages could be used as immunogens either alone as demonstrated here in mice or in combination with other strategies for the induction of MPER specific immunity against HIV-1.
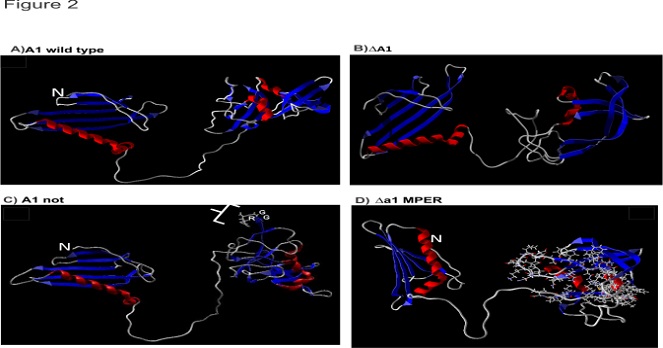
A) A1 wild type (B) ΔA1 (C) A1Not (D) ΔA1MPER (MPER highlighted in ball and stick rendering at the C terminus region of the protein). (N indicates the N terminus of the minor coat protein A1; red and blue colored structures indicate α-helix and β-sheets, respectively)

